
|
by
João
A. N. Batista (1), Luciano de Bem Bianchetti(1) & Eduardo G. Gonçalves(2) |
| (1) Embrapa Recursos Genéticos
e Biotecnologia, Parque Estação Biológica, Final Av. W5 Norte, CP 02372, Brasília-DF, 70770-901, Brazil janb@cenargen.embrapa.br bianchet@cenargen.embrapa.br (2) Universidade de São Paulo, Dept. de Botânica, CP 11461, São Paulo-SP, 05422-970, Brazsil edggon@hotmail.com |
| Trabalho publicado originalmente na Novon, revista de nomenclatura botânica do Missouri Botanical Garden, volume 13, número 4, páginas 397 a 402, em 2003. |
ABSTRACT.
Habenaria pabstii, a new species (Orchideae, Orchidaceae) from the core region of the cerrado vegetation of central Brazil, is described and illustrated. The species has been collected since 1961, but was repeatedly misidentified as other taxa. The species is similar to those in section Macroceratitae, but distinct by the shorter, forward arched, free spur and the shorter, non-involute stigmas. |
RESUMO.
Habenaria pabstii, uma nova espécie (Orchideae, Orchidaceae) da região nuclear do cerrado do Brasil central é descrita e ilustrada. A espécie tem sido coletada desde 1961, mas foi repetidamente identificada erradamente. A espécie é similar àquelas da seção Macroceratitae, mas distinta pelo calcar mais curto, livre, projetado para frente e pelos estigmas mais curtos e não involutos. |
| Key words: Habenaria, new species, cerrado, Brazil. |
|
Habenaria
is the second largest orchid genus in Brazil, with about 165 species
according to the last major survey of Brazilian orchids (Pabst &
Dungs, 1975). The genus is composed exclusively of terrestrial species
and is typical of open grasslands. Most of the species have small, green,
inconspicuous flowers that are of little interest to cultivators and
thus, rarely seen in cultivation. As a consequence, work on the genus
has relied almost completely on botanical collections and on the study
of dried material. |
 figure 2A |
The three major treatments of the genus for the country so far are those
of Cogniaux (1893) in Flora Brasiliensis, Hoehne (1940) in Flora
Brasilica and Pabst & Dungs (1975, 1977) in Orchidaceae Brasilienses.
The large number of species, the poor characterization or little information
on many taxa and the existence of several species complexes, has given
rise to a situation where identification of the species is often troublesome,
with different names being applied to the same species and some species
being overlooked. The main center of diversity of Habenaria in Brazil is the cerrado in the central part of the country. The cerrado is a species-rich savanna vegetation covering 2 million km² of Central Brazil (Ratter et al. 1997). Among the Habenaria species-rich areas in central Brazil is the core region of the cerrado vegetation, including the “Distrito Federal” [Federal District] where 77 taxa are known (Batista & Bianchetti, 2003) and neighboring areas in the state of Goiás. In the course of the survey of the orchid flora of this cerrado region, several taxa of Habenaria have been collected that we have been unable to assign to any known New World species in the genus, one of which, with large and showy flowers is hereby described as new. |
Habenaria
pabstii J.A.N.Bat. & Bianchetti, sp. nov. TYPE: Brazil. Distrito
Federal: Brasília, Fazenda Água Limpa (UnB), 15o57´S-47o55´W,
1070 m, Córrego da Onça, 2 Fev. 1994, L.B. Bianchetti,
J.A.N Batista & B.M.T. Walter 1489 (holotype, CEN; isotypes,
HB, K, MO, SP). Figures 1 & 2 |
Habenaria
pabstii florum generali aspectu speciebus sect. Macroceratitae Kraenzlin
similis sed spora breviori, libera, frontaliter arcuata et stigmatibus
brevioribus, non involutis differt. Etiam H. urbaniana Cogniaux similis
sed floribus frequenter longioribus et structura columnae prominenti
lobo mediano rostelli praedito appendicem similitudine cavernae formans,
haec ultra antheras producta differt. |
|
Terrestrial
herb. Tuber basal, oblong to subspheric, 1.2-2.8 x 0.8-1.7 cm;
roots ca. 1 mm wide, sparsely hairy, whitened; stem erect, leafy,
20-60 x 0.3-0.4 cm including the inflorescence. Leaves 7 to 11,
the 3 or 4 lowermost sheath-like, the largest around the center of stem,
(3)4 to 5(6), spread, linear-lanceolate, 11-21 x 1-1.4 cm, acute, the
1(2) upper leaves decreasing in size and similar to the lower bracts.
Inflorescence 5-10 cm long, few-flowered; bracts ovate-lanceolate,
1.8-3.1 x 0.8-1 cm, caudate, the lowermost almost the same size as the
pedicellate ovary, decreasing in size towards the apex of the inflorescence,
green. Flowers (1)2 to 5(7), spreading, superimposed, large for
the genus, greenwhite; pedicellate ovary, 2.5-3.5 cm long, slightly
arched, green; sepals mucronate, light green with the margins whitened,
the veins slightly marked in darker green; dorsal sepal concave, when
flattened somewhat elliptic, 12-16 x 8-13 mm; lateral sepals obliquely
oblong, 16-20 x 4.5-6.5 mm, acute, deflexed in fully open flowers; petals
bipartite, base white or creamy white, green towards the apex; posterior
segment linear-falcate, 13-15 x 2-3 mm, acute, base free, towards the
apex connivent with the dorsal sepal; anterior segment linear-filiform,
25-43 mm long, 2-2.5(2.8) times longer than the posterior segment, projected
forward and somewhat parallel to the lip lateral segments; lip tripartite,
projected forward, base white or creamy white and light green towards
the apex of the segments; undivided base 2-3 x 2-3 mm; lateral segments
linear-filiform, 32-51 mm long, 1.7-2.3 times longer than the medium
segment; medium segment shorter and wider, linear-ligulate, 17-24 x
1.5(2) mm, the apex bent upward; spur pendent, clavate, free from the
bracts, slightly to strongly arched forwards, narrower at the base,
progressively thickened towards the apex, 3.3-3.8 cm long, base 1.5
mm wide, apex 2.5-3 mm wide, green; column 6-10 mm high; anthers ca.
4-6 mm tall, white-yellow; anther-canals 7-9 mm long, slender, erect,
somewhat perpendicular to the anthers; connective emarginate, green;
rostellum mid-lobe prominent, elongate, forming a tunnel-like structure
projected beyond the anthers, ca. 10-11 mm long including the lateral
arms, 6-7 mm tall at the apex, fleshy, cuspidate, acute, light-green,
the base white, slightly bent, forming a discrete groove that extends
through the lateral arms and which supports the anther canals; rostellum
arms white, 3-4 mm long; auricles erect, ca. 2.5-3 mm tall, fleshy,
flattened, verrucose towards the apex, white; stigmas 2, closely parallel,
6-8 mm long including the base, the receptive, exposed surface 3-4 mm
long and 2-2.5 mm wide, projected forward, non-involute, upper surface
flatly convex, light green; pollinaria 2, yellow, viscidium ca. 1 x
1 mm, spaced 3-5 mm apart; caudicles elongate, 8-10 mm long, about 2-3
mm from the apex ca. 90o bent; pollinia 4, 3-4 x 1-1.3 mm, laterally
flattened, perpendicular to the caudicles main axis. Fruit not
examined. |
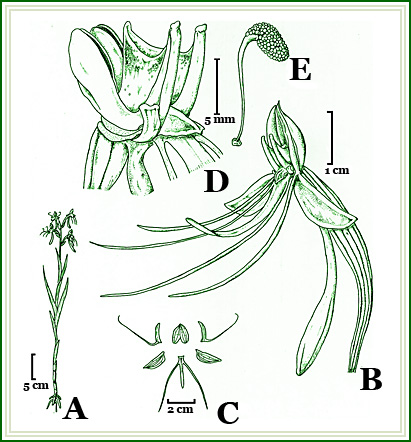 Figure 1. Habenaria pabstii Batista & Bianchetti. A. Habit, B. Flower - 3/4 view, C. Floral diagram, D. Column - 3/4 view, E. Pollinaria. Drawn from the type collection, Bianchetti et al. 1489, by Eduardo Gonçalves. |
|
Etymology.
The new species is named in honor of Guido F.J. Pabst (1914-1980), the late well known Brazilian orchidologist, author of Orchidaceae Brasilienses, who greatly contributed to the knowledge of the orchid flora of central Brazil and who described some new species of Habenaria. Distribution. Habenaria pabstii is an occasional species, so far only known from the Distrito Federal and the state of Goiás. It should also be expected in western Minas Gerais, which is floristically related to this core cerrado region, which shares many orchid species in common with the Distrito Federal and Goiás, and probably also in southern Tocantins state, which is close to the known range of the new species. Habitat, Ecology and Phenology. Habenaria pabstii is more commonly found in seasonally wet grassy fields, in either hydromorphic or sandy soils. Occasionally, it can also be found growing under drier conditions or, rarely, in permanently wet ground with water at the surface. Like most Habenaria species in central Brazil, flowering occurs during the rainy season, from December to late March, extending from the peak to almost the end of the rainy season. Plants frequently appear in great numbers, with apparently only the older and more developed individuals flowering among many still growing younger plants. The flowers usually do not last more than a few days in the field (pers. obs.). Also, like many other Habenaria and terrestrial orchids from open grasslands, the flowering of this species is greatly enhanced by bushfires (Jones, 1993; Oliveira et al. 1996). The greatest concentration of flowering plants are usually found in burnt areas, though occasionally some plants can also be found in flower in unburnt places (pers. obs.). The pollination mechanism and pollinators of the species are unknown. |
 |
Habenaria
pabstii was apparently first collected by Ezechias P. Heringer in
1961 in the Distrito Federal. Analysis of dried specimens collected
at that time and examined by Guido F.J. Pabst, has revealed that Pabst
misidentified the species as H. fastor Warming, H. candolleana
Cogniaux, H. urbaniana Cogniaux and H. vaupellii Reichenbach.fil.
& Warming. These mistakes probably avoided before the identification
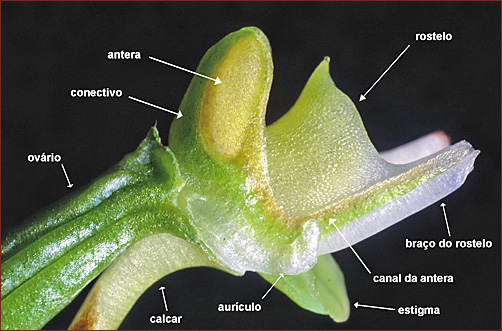
of H. pabstii as a new species. Amongst Brazilian Habenaria, H. pabstii is mostly similar in general flower morphology and size to a species group within section Macroceratitae Kraenzlin, represented by H. bractescens Lindley, H. fastor Warming, H. gourlieana Gillies ex Lindley, H. johanennsis Barbosa Rodrigues, H. longicauda Hooker and H. macronectar (Vellozo) Hoehne, hence prior Pabst identifications as H. fastor and H. vaupellii (a synonym of H. johannensis). However, distinct from all these species, H. pabstii has a shorter, forward-arched free spur (Figure 1B, 2A) and shorter, non-involute stigmas, which are closely parallel forming a single receptive surface turned up and frontward (Figure 1D, 2B). All these other species have pendent spurs, which are completely or partially enclosed by the bracts and which are longer than the ovary and pedicel, ranging from 4 to more than 15 cm, and reaching up to 25 cm in H. longicauda (Snuverink & Westra, 1983). Also, their stigmas are always longer (the free part 6-9 mm long in other species with similar flower size), separated and in contact only at the apex, the margins involute, that is, rolled inwards spirally on each side (Stearn, 1983) and the receptive surface at the apex mostly turned to the sides. Thus, though H. pabstii undoubtedly shares some degree of similarity with these species, it differs in some important details of the column structure and its exact infrageneric classification is unclear. As current infrageneric classification of Habenaria needs a radical reassessment (Pridgeon et al. 2001), further studies, including a molecular analysis, will be necessary to clarify the relationship between H. pabstii, the species in sect. Macroceratitae and other species in the genus. Among other species in sect. Macroceratitae, H. pabstii is also similar in general flower morphology to H. quinqueseta (Michx.) A. Eaton. However, this species has the leaves more patent, ovate to lanceolate and broader (2-6 cm wide); petals and lip white; a longer, pendent spur (5-10 cm long, reaching up to 18 cm in H. quinqueseta var. macroceratitis (Willd.) Luer); and the rostellum mid lobe smaller, completely placed between the anther-sacs (Luer, 1972; Correll, 1978). Additionally, H. quinqueseta has a distinct distribution, occurring in Southern North America, Central America, the Antilles and Northern South America, and has never been recorded and is not know to occur in Brazil. Beyond the species in sect. Macroceratitae the only other Brazilian species to which H. pabstii is similar and could be confused is H. urbaniana (sect. Nudae Cogniaux). Both species are similar in the large flowers and long and filiform lateral segments of the petals and lip. However H. urbaniana is vegetatively taller (86-122 cm high), has lanceolate leaves up to 2 cm wide; a longer (12-19 cm) and many-flowered inflorescence ((6)12 to 25 flowers) with overall smaller flowers with proportionally less developed anterior petals (petals anterior segment 19-33 mm long, 1.6-1.9 times longer than the posterior segment); a shorter floral spur (ca. 2-2.5 cm) which at most equals the ovary plus its pedicel in length, and prominently broadened at the apex (1 mm wide at the base, 3-4 mm wide in the apex). Additionally, the column structure between both species is very different, with the middle lobe of the rostellum of H. urbaniana smaller (about 1.5 mm long and 2 mm tall), triangular and completely enclosed by the anthers. Lastly, plants of H. pabstii became black when dried or preserved in ethanol, while those of H. urbaniana and the species in sect. Macroceratitae became brown. The identification as H. candolleana is unwarranted since this species has much smaller flowers (dorsal sepal and lip lateral segments 6-8 mm long), and a very distinct column structure. In column structure, mainly in relation to the rostellum morphology, H. pabstii is very similar to H. johannensis. In both species, the rostellum mid-lobe forms a prominent tunnel-like structure, which is partially erect and projected beyond the anthers (Figure 2B-C). Despite this morphological similarity, it is not known, and would be interesting to investigate, if both species share any similarities in their mechanisms of pollination. |
 figure 2B - column |
 figure 2C - rostellum |
Paratypes.
BRAZIL. Acknowledgments.
|
|
- Batista,
J. A. N. & L. B. Bianchetti. 2003. Lista atualizada das Orchidaceae
do Distrito Federal, Brasil. Acta Bot. Bras. 17(2): 183-201. |
|