
Brittonia, vol 56 (3) - 260.274,
2004
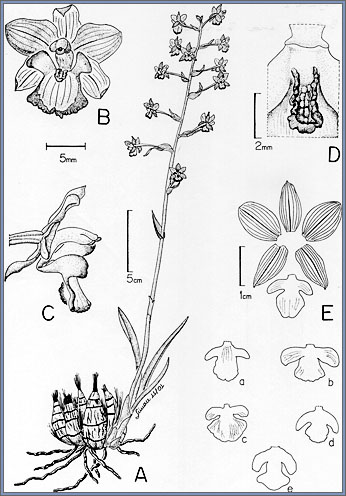
Fig. 1 |
Cyrtopodium
brunneum
A.
Habit at anthesis.
B. Frontal view of flower.
C. Side view of flower.
D. Callus.
E. Perianth.
a-e. Variations in lip morphology, each one from a different
individual of the same population.
A-D drawn from Batista 1242,
E and a-e drawn from Batista 208 by Simone C. Souza e Silva. |
ABSTRACT. Cyrtopodium
brunneum, C. lamellaticallosum and C. poecilum
var. roseum, two new species and a new variety from the
cerrado and campo rupestrian vegetation of central and southeastern
Brazil are described and illustrated. Although the description
of Cyrtopodium gonzalezii indicates that it is a distinct
species, the holotype is referable to C. brandonianum.
The undescribed plants are now described here as Cyrtopodium
brunneum and C. gonzalezii is placed in the synonym
of C. brandonianum.
Key
words: Orchidaceae, Cyrtopodium, cerrado vegetation,
Brazil.
Cyrtopodium
is a Neotropical genus with about 42 species ranging from southern
Florida to northern Argentina. The center of diversity of the
genus is in the Brazilian cerrado, where at least 25 species occur.
In recent years, several new species have been described in the
genus (Bianchetti & Batista, 2000; Batista & Bianchetti,
2001; Menezes, 2000), most from central Brazil. Based on our further
exploration of the region and examination of herbarium specimens
of the genus, we describe bellow two new species C. brunneum
and Cyrtopodium lamellaticallosum and and a new variety, C.
poecilum var. roseum.
Traditionally, the taxonomy of Cyrtopodium has been based
almost completely on reproductive characters, with the vegetative
parts, particularly the leaves, being neglected. This is because
botanical studies of the genus have relied almost entirely on
dried specimens collected mainly during the flowering season,
when most Cyrtopodium species do not have fully developed
leaves. The examination of live plants throughout the developmental
cycle has allowed characterization of the vegetative parts of
most species and, in many cases, the species can be identified
vegetatively. Based on this characterization, we found that the
holotype of C. gonzalezii L. C. Menezes is unambiguously
C. brandonianum Barb. Rodr., although the description does
indicate that it is a new species. This new species is described
here as C. brunneum.
Type:
BRAZIL. Distrito Federal, Brasília, Plano Piloto, final
da Asa Norte, area between the "Parque Ecológico Norte"
and "Parque Nacional de Brasília", the futur
"Setor Noroeste", 8 Sep 2001 (fl), J. A. N. Batista
1242 (HOLOTYPE: CEN; ISOTYPES: AMES, BHCB, HB, ESA, HUEFS, HUFU,
K, MBM, MO, NY, RB, SP, SPF, UB, UEC).
Cyrtopodium
gonzalezii L.C.Menezes in Boletim CAOB 6(1): 9. 1995. pro
parte, excluding type.
Cyrtopodio
tristi Rchb. f. & Warm. similis sed inflorescentia semper
simplici, floribus minoribus, sepalis oblongo-lanceolatis vel
paulo lanceolato-ovatis, labelli lobis lateralibus falcatis differt;
etiam C. dusenii Schltr. similis sed foliis sub anthesi
redactis, inflorescentia semper simplici, floribus majoribus,
sepalis et petalis atrobrunneis differt.
Terrestrial herb. Roots many, whitish, glabrous. Pseudobulbs completely
buried, ovoid, leafless from the second year onwards, externally
white, (3-)4-6(-7) x (1-)1.5-2.5 cm. Leaves at flowering 3-6,
little developed, 7-15 cm long, when fully developed 5-8, spreading,
coriaceous, lanceolate, the lowermost 1-2 sheath-like, the uppermost
4-7 13-33 x 1-2.6 cm, articulate 1-2 cm from the surface of the
soil, the apex acuminate. Inflorescence lateral, erect, racemose,
lax, 26-56 cm, brownish to dark brown; peduncle 11-26 cm, the
2 sheathlike bracts strongly adpressed, 1.7-3.5 cm long; rachis
12-26 cm long; floral bracts oblong-lanceolate, 1-3 x 0.5-0.9
cm, greenish brown, acute to acuminate, the margins undulate,
incurved; ovary with pedicel 1.4-3.9 cm long, dark brown. Flowers
9-15(-20) per inflorescence, sweet-scented. Sepals oblong-lanceolate
to slightly lanceolate-ovate, light to dark brownish or brownish
green, lighter at the base, frequently with dark-brown spots,
the apex apiculate, the margin undulate; dorsal sepal (8-)11-13(-15)
x 4-5(-6) mm; lateral sepals slightly oblique, (8-)11-13(-15)
x 4-6(-7) mm. Petals concave, broadly elliptic to somewhat broadly
lanceolate, (8-)9-12(-13) x 6-8 mm, the lower half greenish to
light-brownish, occasionally with few to many small brown dots,
the upper half dark-brown, color transition usually sharply delimited,
the apex obtuse to rounded, slightly apiculate, the margins slightly
undulate. Lip 3-lobed, (7-)9-11(-12) mm long, 10-12(-14) mm wide
between the apices of the side lobes when spread, the base shortly
unguiculate, 1 mm long, yellowish; lateral lobes erect, parallel,
oblong-falcate, (3-)4-5(-6) x 2-3(-4) mm, reddish to reddish-purple,
the apex obtuse, the base unconstricted, the margins entire, smooth;
callus verrucose, slightly sulcate, extending from the base of
the central lobe to the column foot, white, occasionally reddish-white;
isthmus separating the lateral lobes from the central lobe usually
prominent, sometimes elongated, 1-2(-3) mm long; central lobe
variable, obcordate to obovate-oblong to somewhat obreniform,
5-7 x (5-)6-8(-10) mm, yellow with red to reddish-purple margins,
occasionally with a few red dots at the base, the yellow becoming
fainter towards aging, the apex with a protruding fold, retuse
when flattened, the margin smooth, occasionally slightly verrucose
at the apex. Column erect, slightly arcuate, trigonous, 6-7(-8)
mm long, base yellow, middle reddish white and greenish purple
towards the apex; column foot (2.5-)3-4 mm long, yellow at the
base, white at the middle with small numerous red dots that merge
towards the apex. Anther ca. 2-2.5 x 1.5 mm, yellowish, apex green;
pollinia 2, waxy, sulcate, ca. 1 x 0.5-0.6 mm, yellow; stipe triangular,
hyaline, ca. 1 x 1 mm at base. Fruit deflexed, green, oblong to
elongate, sulcate, ca. 6-8.5 x 1-1.5 cm when young, including
the pedicel.
Etymology. - Named after the dark brown color of the sepals and
petals.
Additional specimens examined. BRAZIL. Distrito Federal: Brasília,
Vila Maury, 6 Sep 1960 (fl), Andrade 412 & Emmerich 404 (HB,
R); Brasília, Lago Norte, 8 Oct 1990 (fl), Batista 122
(CEN), 1 Oct 1991 (fl), Batista 208 (CEN), 7 Sep 1992 (fl), Batista
325 (CEN); Guará, Reserva Ecológica do Guará,
13 Sep 1992 (fl), Batista 330 (CEN); Brasília, area between
Parque Ecológico Norte and Parque Nacional de Brasília,
7 Oct 1994 (fl), Batista 421 (CEN), 11 Dec 1994 (veg), Batista
434 (CEN); Brasília, Lago Norte, 17 Feb. 1995 (veg), Batista
521 (CEN), QL 15, near Clube do Congresso, 17 Feb. 1995 (veg),
Batista 523 (CEN); Núcleo Bandeirante, Santuário
Ecológico do Riacho Fundo, 14 Sep 1995 (fl), Batista &
Bianchetti 576 (CEN); Brasília, area between Parque Ecológico
Norte and Parque Nacional de Brasília, 28 Sep 1998 (fl),
Batista 792 (CEN, HB, MBM, SP), 30 Dec 2000 (veg), Batista 1112
(CEN); Brasília, area between Lago Norte and EPIA, 6 Jan.
2001 (veg), Batista 1126 (CEN); Brasília, Lago Sul, condomínio
Prive Morada Sul II, above QI-28, close to Paranoá dam,
(fl. in cult.) 22 Sep 2001, Batista et al. 1184 (CEN); Gama, BR-060,
near the GDF check point and the access to Santo Antônio
do Descoberto, 2 Oct 2001 (fl), Batista & Oliveira-Neto 1252
(CEN); Brasília, Plano Piloto, Asa Norte, 713-714 N, area
of University of Brasília, between Parque Olhos D´Água
and the CAESB sewage treatment plant, 7 Oct 2001 (fl), Batista
1254 (CEN); Brasília, Setor de Mansões do Lago Norte,
17 Sep 1990 (fl), Bianchetti & Batista 953 (CEN), trecho 2,
20 Oct 1991 (fl), Bianchetti & Batista 1176 (CEN), near MI-7,
6 Oct 1991 (fl), Bianchetti & Batista 1168 (CEN); Guará,
Reserva Ecológica do Guará, 26 Aug 1990 (fl), Bianchetti
& Batista 950 (CEN); Brasília, Plano Piloto, 10 Nov
1961 (fl), Heringer 8756 (HB, UB); Brasília, Estação
Florestal Cabeça do Veado, 8 Oct 1965 (fl), Heringer 10616
(HB, UB); Santa Maria, near the microwave towers, 28 Oct 1972
(fl), Heringer 12206 (HB, UB); ca. 20 km S of Brasília,
on rd. to Goiânia, near Rio Melchior, 1125 m, 25 Sep 1965
(fl), Irwin et al. 8652 (UB); Brasília, confluence of Rio
Torto with Lake Paranoá, 975 m, 9 Oct 1965 (fl), Irwin
et al. 9086 (HB); Guará, Reserva Ecológica do Guará,
15o50’S, 47o57’W, 1050 m, 20 Sep 1994 (fl), Oliveira
2 (UB). Goiás: Chapada dos Veadeiros, ca. 26 km N from
Alto Paraíso, GO-118, 11 Oct 1999 (fl), Batista 945 (CEN);
ca. 8 km from Niquelândia, Tocantins Niquel Company, 14o23’48”S,
48o25’59”W, 17 Sep 1996 (fl), Fonseca et al. 1154
(IBGE); Municipality of Mineiros, BR-364, near Córrego
Alegre, 20 Sep 1974 (fl), Hatschbach & Kummrow 35014 (MBM);
Goiânia, 30 Nov 1963 (fl), Heringer 9289 (HB); Municipality
of Luziânia, 16 Sep 1974 (fl), Heringer 13971 (HB, UB);
12 km NW of Veadeiros, on rd. to Cavalcante, 1200 m, 21 Oct 1965
(fl), Irwin et al. 9444 (HB, UB); Aparecida de Goiânia/Hidrolândia,
10 Aug 2002 (fl), Pastore 32 (CEN). Mato Grosso: 84 km from Alto
Araguaia, towards Rondonópolis, BR-364, Sep-Nov 1983 (fl),
Hutchison 8550 (UEC). Minas Gerais: Uberlândia, Estação
Ecológica do Panga, 24 Sep 1992 (fl), Araujo et al. 276
(HUFU); Morro Pelado, near Campanha, Aug 1896 (fl), Brandão
in CGGMG 1719 (R) (p.p., with C. cristatum); Municipality
of Carmo do Rio Claro, Serra da Tormenta, 3 Nov 1990 (fl), Campos
s.n. (CEN, HRCB); Serra do Cipó, 23 Aug 1958 (fl), Heringer
6438 (HB) (p.p., with C. parviflorum); Felixlândia,
Três Marias basin, 23 Aug 1958 (fl), Heringer 6438 (UB),
2 Oct 1959 (fl), Heringer 6438 (UB), margins of Rio Paraopeba,
11 Oct 1959 (fl), Heringer 6438 (UB); Santana do Riacho, km 107
on the Belo Horizonte to Conceição do Mato Dentro
rd., 4 Oct 1981 (fl), Pirani et al. in CFSC 7472 (SP, SPF).
Pabst
was probably the first orchid taxonomist to examine material of
C. brunneum, but misidentified it first as C. falcilobum
Hoehne & Schltr. (now a synonym of C. parviflorum
Lindl.) and latter as C. poecilum Rchb. f.& Warm. f.
minor Hoehne (now a synonym of C. fowliei L.C.Menezes).
He was apparently also the first taxonomist to suspect that the
specimens might represent a new species. In his personal files
at the Herbarium Bradeanum, there is a card with a sketch of C.
brunneum indicated at the side as C. aff. parviflorum
and at the top as Cyrtopodium heringeri; but he never published
a valid description of this species. More recently, Menezes described
this species as Cyrtopodium gonzalezii L.C.Menezes (Menezes,
1995), but unfortunately designated a holotype that is Cyrtopodium
brandonianum Barb. Rodr. This situation leaves the new species
without valid publication.
The holotype of C. gonzalezii, at the University of Brasília
herbarium is a single, sterile specimen. However, on the basis
of its vegetative characteristics this specimen is C. brandonianum.
The holotype has small, narrow, buried pseudobulbs and linear,
erect, very narrow leaves. Compared with the 25 Cyrtopodium
species known from the cerrado, the buried pseudobulb characteristic
excludes several species with exposed pseudobulbs, such C.
aliciae Linden & Rolfe, C. cardiochilum Lindl.,
C. cipoense L.C.Menezes, C. cristatum Lindl., Cyrtopodium
eugenii Rchb. f., C. hatschbachii Pabst, C. lissochiloides
Hoehne & Schltr., C. palmifrons Rchb. f.& Warm.,
C. paludicolum Hoehne, C. parviflorum Lindl., C.
saintlegerianum Rchb. f., C. virescens Rchb. f.&
Warm., and C. vernum Rchb. f.& Warm.
According to the protologue of C. gonzalezii (Menezes,
1995) and to Menezes herself (L. C. Menezes, pers. comm.), the
holotype was collected on the peninsula do Lago Norte, a district
of Brasília where we have collected intensively during
the past 12 years. Six species of Cyrtopodium are known
from this district: Cyrtopodium blanchetii Rchb. f., C.
brandonianum Barb. Rodr., C. brunneum, C. caiapoense
L.C.Menezes, Cyrtopodium poecilum Rchb. f.& Warm.
and C. virescens Rchb. f.& Warm. This further excludes
from the comparison those species that do not occur in the Distrito
Federal, such as C. braemii L.C. Menezes and Cyrtopodium
dusenii Schltr., or those that occur in Brasília but
not at this particular site, such as C. fowliei L.C.Menezes,
C. latifolium Bianchetti & J.A.N.Bat., C. pallidum
Rchb. f.& Warm., C. triste Rchb. f.& Warm.
and C. linearifolium J.A.N.Bat. & Bianchetti. Additionally,
the pseudobulbs of C. braemii, C. fowliei and C.
latifolium are reddish-purple externally, while in the holotype
of C. gonzalezii they are whitish.
The characterization of the vegetative parts of the remaining
species for comparison, including the holotype of Cyrtopodium
gonzalezii, is shown in Table I. Because of its similarity
to C. brunneum, C. triste is also included in the
comparison.
Table
1
Vegetative characteristics in seven terrestrial species
of
Cyrtopodium known from the Lago Norte district of
Brasília, Brazil.
|
|
C.
brunneum |
C.
triste |
C.
brandonianum |
C.
gonzalezii (holotype) |
C.
poecilum |
C.
banchetii |
C.
caiapoense |
Pseudobulbs
|
Length
(cm) |
(4)5-8.8(7.5) |
(3)4-6 |
3.5-5.5(6.5) |
3.5-4 |
(5)6-8(11.5) |
(4.5)5-8(8.5) |
7.5-8 |
Width
(cm) |
(1.4)1.7-2.3(2.8) |
1-2.3 |
0.8-1.5(2) |
1.2-1.3 |
1.5-2.8 |
1.5-2.5(3.5) |
0.8-1.2 |
Position |
buried |
buried |
buried |
buried |
burieds |
buried |
buried |
Color |
white |
white |
white |
white |
reddish-purple |
white |
white |
Leaves |
Number |
(4)5-7(8) |
(3)4-5(6) |
(3)4-5(7) |
5 |
(3)5-6 |
(3)4-5(6) |
(6)8-10 |
Length
(cm) |
(6)16-25(33) |
(5)12-21(31) |
|
|
|
|
|
Width
(cm) |
(0.6)1.1-2.1(2.6) |
(0.8)1.1-1.6(2.0) |
(0.7)1-1.5(2.1) |
0.5-0.9 |
(1.3)2.5-3.5(5.1) |
(1.3)2-2.9(3.4) |
1.2-2.7 |
Ratio
L/Wa |
(8.1)9.5-15.5(17.2) |
12.9-15.5(21.9) |
(31.2)37-42(60) |
55 |
(12.7)16.5-18.5(30) |
(11.6)18.5-25(33.2) |
15.3-18.5(21.6) |
Ratio
L/W b |
(6.7)8.5-16.7(22) |
(7.2)10-21(29.3) |
(15)30-58(76) |
55 |
(7.6)11.3-20.8(30.6) |
(8.8)14-30(47.2) |
10-22(31.7) |
Shape |
lanceolate |
linear-lanceolate |
linear |
linear |
lanceolate |
lanceolate |
lanceolate |
Position |
spreading |
spreading |
erect |
erect |
spreading |
erect |
erect |
Articulation |
present |
present |
present |
present |
present |
present |
present |
Length
(cm) |
(0.5)1-2(3) |
(0.5)1-2(2.5) |
(2.5)4-6(10.5) |
4-4.5 |
(2.5)3.5-6.5(8) |
(3)4.5-6.5(8) |
2-3.5(6) |
| a:
mean ratio per plant
b: ratio per leaf |
|
All measurements were taken from living specimens under field
conditions, except for the measures of pseudobulbs and the holotype
of C. gonzalezii, which were obtained from dried specimens.
Only mature and adult specimens and fully developed leaves were
considered in the analysis. Leaf length was measured from the
surface of the soil, which corresponds approximately to the distance
from the apex of the pseudobulb to the apex of the leaf.

Fig.
7. Cyrtopodium poecilum. Fully developed (mature)
leaves. Plant in campo sujo vegetation. |
Cyrtopodium
poecilum (Fig. 7) and C. blanchetii (Fig. 8) have
much broader leaves than Cyrtopodium gonzalezii. |

Fig.
8. Cyrtopodium blanchetii. Plant with mature leaves
and fruits. |

Fig. 9. Cyrtopodium poecilum.
Pseudobulbs. |

Fig. 10. Cyrtopodium blanchetii.
Pseudobulbs. |

Fig. 11. Cyrtopodium caiapoense.
Vegetative parts |
The pseudobulbs of C. poecilum are reddish purple externally
(Fig. 9), and therefore distinct from all the other species in
this comparison; the pseudobulbs of C. blanchetii (Fig.
10) are larger than those of C. gonzalezii.
Cyrtopodium caiapoense (Fig. 11) has more and wider leaves
and usually has longer pseudobulbs than Cyrtopodium gonzalezii,
On the other hand, C. brunneum and C. triste has
shorter and broader leaves. In summary, the vegetative parts of
neither of the five previous species is compatible with the holotype
of Cyrtopodium gonzalezii (Table 1).
Specifically comparing C. brunneum and C. gonzalezii,
the mean leaf length-to-width ratio was 9.5-15.5 for Cyrtopodium
brunneum, and 55 for C. gonzalezii (Table 1). Additionally,
the leaves of C. brunneum are typically spreading (Figs.
4-5), while in C. gonzalezii, they are erect.
 Fig.
5a. Cyrtopodium brunneum. Uprooted mature plant (January),
showing the fully developed leaves and underground pseudobulbs.
Lateral view.
Fig.
5a. Cyrtopodium brunneum. Uprooted mature plant (January),
showing the fully developed leaves and underground pseudobulbs.
Lateral view. |
 Fig.
4. C. brunneum. Fully developed leaves, in December,
about 2 months after flowering. The surrounding vegetation
has been removed to expose the plants.
Fig.
4. C. brunneum. Fully developed leaves, in December,
about 2 months after flowering. The surrounding vegetation
has been removed to expose the plants. |
 Fig.
5b Cyrtopodium brunneum. Uprooted mature plant (January),
showing the fully developed leaves and underground pseudobulbs.
Front frontal.
Fig.
5b Cyrtopodium brunneum. Uprooted mature plant (January),
showing the fully developed leaves and underground pseudobulbs.
Front frontal. |
The
pseudobulbs of C. brunneum are usually slightly longer
and broader than those of C. gonzalezii. Another difference
involves the distance of the leaf articulation relative to the
apex of the pseudobulb, which was shorter in C. brunneum than
in C. gonzalezii.
 Fig.
12. Cyrtopodium brandonianum. Plant with fully developed
leaves.
Fig.
12. Cyrtopodium brandonianum. Plant with fully developed
leaves. |
Of
the species presented in table 1 the only that has characteristics
compatible with the holotype of Cyrtopodium gonzalezii
is Cyrtopodium brandonianum (Figs. 12-13). |
 Fig.
13. Cyrtopodium brandonianum. Pseudobulbs.
Fig.
13. Cyrtopodium brandonianum. Pseudobulbs. |
To analyze these similarities in greater details a plot of leaf
length versus width for C. brunneum, the holotype of C.
gonzalezii and C. brandonianum is shown in Figure 14.
These results show that the vegetative characteristics of the
holotype of C. gonzalezii are distinct from those of Cyrtopodium
brunneum, C. poecilum, C. blanchetii and C.
caiapoense, but falls within the range of variation of C.
brandonianum.
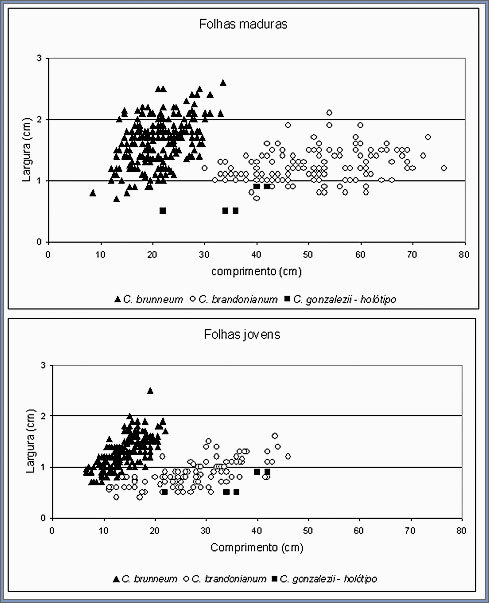
Fig.
14. Plot of leaf length vs. width for mature, fully developed
leaves and young, incompletely developed leaves of C.
brunneum, C. brandonianum and the holotype
of C. gonzalezii. Measures of the holotype of C.
gonzalezii are the same and repeated for both mature
and young leaves. |
When compared to C. brandonianum, the slightly smaller
values for C. gonzalezii, particularly for leaf width,
can be explained by the fact that the measurements were taken
from a dried specimen, in which the leaves may have shrunk during
drying. The size and number of pseudobulbs indicate that the type
specimen of C. gonzalezii is most certainly an adult plant,
but with immature leaves. To further examine this possibility,
the length and width of immature leaves of C. brunneum and
C. brandonianum were plotted with the data for the holotype
of Cyrtopodium gonzalezii (Fig. 14). As before, and with
a greater coincidence, the leaf measurements for the holotype
of C. gonzalezii fell within the range of measurements
for C. brandonianum. In agreement with these results, the
collection date for the holotype of C. gonzalezii as indicated
on the holotype label is early November. Most Cyrtopodium
species in central Brazil, flower at the end of the dry season
and the beginning of the rainy season (September to November),
while C. brandonianum flowers mainly during the peak of
the rainy season (December and January). However, In common with
most other species, the vegetative growth of Cyrtopodium brandonianum
starts at the beginning of the rainy season, usually during October,
so that in November, plants have already initiated vegetative
growth but have incompletely developed leaves.
Of the remaining terrestrial species of Cyrtopodium with
small, buried pseudobulbs, only two have long, linear, straight
leaves like the holotype of C. gonzalezii. The first, C.
linearifolium, occurs in dark, sandy-clay soil associated
with campo rupestrian vegetation found at higher altitudes, has
nonarticulated leaves, and, in the Distrito Federal, is known
only from the higher altitude meadows of the Chapada da Contagem.
The second, Cyrtopodium pallidum, occurs at several sites
throughout the Distrito Federal but typically grows in dark, sandy-clay
soil found in wetter areas (close to gallery forests) usually
associated with regularly spaced mounds of earth known as murundus,
and does not occur on the dark red latosol of typical cerrado
of the Lago Norte district.
In summary, we conclude that the type specimen of C. gonzalezii
is an individual of C. brandonianum with leaves that
are not fully developed.
A critical analysis of the description of C. gonzalezii (Menezes,
1995) reveals a mixture of characteristics, with the vegetative
parts corresponding to C. brandonianum and the reproductive
parts to C. brunneum. Menezes (1995) intended to describe
a new species, and, indeed, the pictures of flowers in the original
description are from C. brunneum. However, according to
the International Code of Botanical Nomenclature (Greuter et al.,
2000), the nomenclatural type is that element to which the name
of a taxon is permanently attached, whether as a correct name
or as a synonym. The holotype of C. gonzalezii is C.
brandonianum, thus C. gonzalezii is a synonym of C.
brandonianum.
Cyrtopodium brunneum inhabits the campo limpo and campo sujo
vegetation (Fig. 2), on deep, reddish, clay soil (dark red latosol),
exposed to full sun, being protected only by the surrounding herbaceous
vegetation. This soil is moist for brief periods during the rainy
season, but never retains water for long periods and dries completely
during the dry season. Cyrtopodium poecilum Rchb. f. &
Warm., Cyrtopodium brandonianum Barb. Rodr., C. triste
Rchb. f. & Warm., C. blanchetii Rchb. f., and C.
caiapoense L. C. Menezes all occur in the same habitat and
are frequently found with C. brunneum.

Fig. 2 - Cyrtopodium brunneum.
Habitat at the time of flowering. Burnt cerrado at the type
locality, about 2 weeks after fire, with the vegetation
beginning to regrow, at the beginning of September. |
Less often, C. brunneum is found on rocky, shallow, sandy
clay soil, where it grows with C. vernum Rchb. f. &
Warm and C. cristatum Lindl. Cyrtopodium brunneum
also occurs in the typical cerrado, under partial shade. In all
of these habitats, the pseudobulbs of C. brunneum are completely
buried in the ground, sometimes with only the upper part exposed.
Flowering in Cyrtopodium brunneum extends from the end
of the dry season to the beginning of the rainy season, from late
August to November, but it flowers mainly during September and
October. This flowering coincides with that of C. poecilum,
C. blanchetii, C. caiapoense, C. cristatum and C. vernum; C. triste
usually flowers a little later (late October to early December),
as does C. brandonianum (mainly from December to January).02*

Fig.
3. Cyrtopodium brunneum. Inflorescence. Note the poorly
developed leaves at anthesis. |
As with other terrestrial Cyrtopodium, flowering
is enhanced by fire, and among the species with small,
buried pseudobulbs C. brunneum is usually the first
to flower after a fire. The inflorescence appears a few
days after a fire and the plants are in full bloom 2-3
weeks later (Fig. 3).
Flowering of the species in unburned places is very rare
or infrequent; we have never seen plants flowering at
unburned sites. Cultivated plants can flower without fire,
very infrequently, and only when exposed to hydric stress
and full sun.
The flowers have a slightly sweet scent.
Removal of the pollinarium occurs somewhat frequently,
indicating that deposition on the stigma may be a more
limiting factor than lack of pollinator visits. Fruit
set is low in nature.
The species most closely related to Cyrtopodium brunneum
is C. triste. In their overall vegetative aspects,
C. brunneum and C. triste are very similar
and difficult to separate. |
The main vegetative
differences between the two species are presented in table 1.
In addition, the leaves of Cyrtopodium brunneum
are slightly more slender, flexible, less coriaceous and rarely
crack when bent; have the base laterally compressed; usually turn
light green and yellow before drying, and tend to fall earlier
than those of C. triste. In Cyrtopodium triste the
leaves are slightly more rigid and coriaceous, and usually crack
when bent; have the base flatter than in C. brunneum; and
change little in color and turn from green to brownish during
senescence. However, many of these characteristics are discrete
and depend upon the age and development of the plants. As a result,
separating the two species based only on their vegetative parts
is difficult.
Development
of the leaves of C. brunneum is dependent upon
habitat conditions. Plants growing under shade in typical
cerrado on deep clay soil usually show maximum leaf development
in length and width. Plants growing on rocky slopes, on
sandy, shallow, poor soil, in almost full sun, are much
smaller, and resemble C. triste in leaf length,
but not in width (small specimens of C. triste have
very narrow leaves). The development of the leaves at
anthesis is incipient, and they only become fully developed
1-2 months after flowering.
As with most other Cyrtopodium species of central
Brazil, growth of Cyrtopodium brunneum is
during the rainy season and dormancy is during the dry
season, when the leaves are lost. Plants very frequently
appear in groups, with several specimens (from 3 to more
than 15) growing close to each other (Fig. 15).
Cyrtopodium brunneum and C. triste are also
very similar in terms of flower color. Both species have
dark brownish sepals and petals and a yellowish red lip.
|
|

Fig.
15. Cyrtopodium brunneum. Group of plants. A common
feature of the species. |

Fig.
16 - C. triste. flower |
However,
C. triste frequently has a branched inflorescence
with up to three lateral branches, larger flowers, and
a lip with broadly obovate lateral lobes (Fig. 16).
Cyrtopodium brunneum is also somewhat similar in
overall aspect to C. dusenii Schltr. However, the
leaves of C. dusenii are already well developed
at anthesis, there is usually a branched inflorescence
with (0-)1-2(-3) lateral branches, and the smaller flowers
are completely yellow with brown spots. Cyrtopodium
brunneum also has been confused with C. parviflorum,
which has a similar flower color and falcate lateral lobes
of the lip (Fig. 17 (Fig. 17). |

Fig.
17 - C. parviflorum. Flower |
However, C.
parviflorum has larger and exposed pseudobulbs (7-20 cm long),
nonarticulate leaves, a longer and frequently branched inflorescence
(0.6-1.2 m long and with (0-)2-4 lateral branches), usually larger
flowers, and longer lateral lobes of the lip (8-10 mm long). Cyrtopodium
parviflorum occurs in wet places and, when found in drier
places, is usually from sandy soils in campo rupestrian vegetation;
it is never found in red clay latosol in typical cerrado vegetation.
| Cyrtopodium
brunneum has a relatively constant flower color (Figs.
6a-b), especially when compared to other species such
as C. braemii L. C. Menezes and C. pallidum
Rchb. f. & Warm., which are highly variable in color.
However, Group of plants. A common feature of the species.
brunneum is extremely variable in terms of lip morphology
(Fig. 1E: a-e), particularly the mid-lobe, and is probably
one of the most variable species in the genus for this
character.
Cyrtopodium brunneum is not an uncommon species,
but the buried pseudobulbs and small size of the plants,
which grow almost completely obscured by grasses, can
make finding plants in the field a difficult task. |
 Fig.
6a - C. brunneum. Flower Fig.
6a - C. brunneum. Flower |
 Fig.
6b - -C. brunneum. Flower Fig.
6b - -C. brunneum. Flower |
Plants are most easily located when they are in flower in vegetation
recently burned. The species is found in the states of Goiás,
Mato Grosso, Minas Gerais, and the Distrito Federal, in the central-western,
central and southeastern Brazilian cerrado. The species may also
be expected to occur in the cerrado region of Tocantins and western
Bahia.
Cyrtopodium
brandonianum Barb. Rodr., Gen. Sp. Orchid. 1: 132. 1877. Type:
BRAZIL. Minas Geraes, Capivary, Barbosa Rodrigues s.n. (No original
material is known. Typified by Cribb & Toscano de Brito, 1996:
1: 30. Original illustration by Barbosa Rodrigues, reproduced
in Sprunger, 1996: 1: 332).
Cyrtopodium
gonzalezii L.C.Menezes in Boletim CAOB 6(1): 9. 1995. Typus:
BRASIL. Distrito Federal, península do Lago Norte, 9 Nov.
1994, L. C. Menezes UB-54 (HOLOTYPUS: UB).
| cont. |
 |
Any
kind of reproduction (print, digital or anyone) of any
type of material of this site: texts, layout, photos,
images and others - is strictly
forbidden without previous written permission of the
authors. Any solicitation or information should be done
by the e-mil: bo@sergioaraujo.com |
|