|
Orchid
News # 34
|
|
XIX WOC
|
Another
look at Hoffmannseggella H.G.Jones
by
Francisco E. Miranda
The so-called rupicolous laelias comprise a very natural and uniform group of species that always have been kept separated from all the other groups in the genus Laelia. More recently, the tendency is not to accept the Mexican and the Brazilian laelias as closely related, which lead us to consider the Brazilian species as not pertaining themselves to Laelia, considering that the type species of the genus is Mexican (Laelia speciosa). Taxonomists then think on where to put the four very well delimited groups of Brazilian laelias. Traditionally, since Schlechter (1917) these groups have been accepted as sections of Laelia, treatment followed by almost everybody after him, e.g. Pabst & Dungs (1975). Since there is no intention here to provide detailed historic facts as they are widely available elsewhere (van den Berg, C. and M.W. Chase. 2004), let’s just say that things really started to change with the work of van den Berg, C. and M.W. Chase. (2000), based in new DNA data. Considering this is a new methodology, it is quite obvious that it will take time for those studies to be fine-tuned to the point of being more useful, and so I decided here to define this group based on more traditional and widely accepted aspects such as morphology and ecology. This being such an uniform group, there is no need to look for solutions when there is not a problem in first place. Of course, I might be making the same mistake as Jones when not discussing the other groups in the Brazilian Laeliinae, but then agin this is not the objective here. Instead, I will be presenting evidence of how well this group is delimited and discussing the validity of features that I think are important and others that are frequently used and are essentially bad or dangerous to use. I hope this kind of approach might be useful when studying other different group in the Laeliinae or even out of it.
Why not accepted when first proposed by Jones in 1968:
Hoffmannseggella was
established by Henry Gordon Jones (1968), and for reasons
mentioned below it was never accepted by taxonomists
and growers alike. It is easy to understand growers,
as they don’t like
and tend not to accept lightly any sort of name changes
that will affect well-known cultivated plants. As for
the taxonomists, the situation was different but basically
boils down to:
First, although the
group has always been considered as very distinct from the time Lindley
(1842) put the species into Parviflorae and throughout the history
of Laelia, Jones did a very poor job describing it as he used features
that not all of the species have and also can be readily found in species
of other groups. These essentially were:
- Lip
much narrower than in Laelia (true to some point).
- Petals
similar to sepals (yes, like in several other groups in the Laeliinae).
- Rupicolous
or rarely dendricolous habit (too vague, same for many genera
in the Laeliinae).
- Laelia (Microlaelia) lundii is
not in the group and has all three features, and just this
example was enough for his work not to be taken seriously.
Secondly,
there is a natural resistance to separate one group and
not do anything about the others (in the case here, the
other “Brazilian laelias”).
And finally, the splitting craze that we see today was not
yet in the works by then.
 |
Laelia
(microlaelia) lundii is a good example of an unrelated species that has all the features mentioned by Jones for Hoffmannseggella. It easily shows why his work was not accepted at the time |
Reasons to accept it:
So,
considering that Jones’s features were not good
enough, let’s try to add other ones and better define
the genus:
1- Star-shaped flowers with essentially similar
sepals and petals. This rules out most of the large-flowered
species from other groups.
2- Flowers have a deeply trilobed
lip with well-defined round to elliptic front-lobe. That
rules out the hadrolaelias and the purpurata types.
3-
Flowers have a lip that is moderately to strongly curved
backwards, and this finally rules out Laelia (Microlaelia) lundii and
all other “Brazilian laelias”.
4- Inflorescences
originating from well-developes sheaths (that can be difficult
to see on very small species, but it is there). This alone
rules out all of the hadrolaelias.
5- Plants always with
very short rhizomes, and this rules out all the other “Brazilian
laelias”.
- There are several other features that are unique to the
group, but for delimitation purposes these are more than
enough. It needs to be mentioned though, that all the species
have uniquely large seeds, and this plays an important
role in local distribution.
   |
Hoffmannseggella species groups
This is a good exercise on judging relevant and irrelevant
features. We always have to choose what to use for taxonomy,
and in many cases a lot of information about the species
is needed to give us a fairly good picture of any group.
In the case of Hoffmannseggella, that is what we
can bring to the table:
A- One way to organize the species is to group them by color, and this will give us three basic groups:
1-
Species with yellow flowers.
2-
Species with pink to purple flowers.
3-
Species with orange to red flowers.
Advantages:
-
Easy to group because it is very easy to see.
Disadvantages:
-
Doesn’t really group species by affinity (with exceptions),
and thus doesn’t help us understand what happens
in nature. This is a
typical artificial grouping, and can be significantly
improved.
B-
Another way to organize the species is by flower morphology,
which in most cases agree with the plant morphology
but not always with flower color. If we want to use
more info, we can add inflorescence habit and, why
not, plant habit. All this can be crossed with geographic
distribution and population variability data, so we
can end up with some pretty solid groups.
Advantages:
-
Allow us to group species by theoretical common ancestor, considering
of course that these group ancestors came from one (if
we consider this a monophyletic group, something that
can be done quite comfortably).
Disadvantages:
-
There is a need for a lot of data to give us a really
good picture of these
groups.
-
Sometimes it is not easy to place species and affinities,
regardless of how much info we have. This means that
the more
info we gather, the more difficult it can get to organize
it.
C-
Some features are of little use or we have to be careful
with, while others are just plain misconceptions.
So, we have to be aware when using them in species
keys. Among these are:
1-
Size of plants:
This
is mostly useless in the group for particular species,
as the same species can have a big variation in
size depending on habitat or growing condition. In the
habitat, light and wind
exposure plays a big role on plant size. The same is true
for plants in collections, where
the impossibility to give proper exposure as in the habitat
produces very different plant.
For example, plants of Hoffmannseggella bradei frequently
get taller in cultivation as
it is difficult to give the high amount of light plants
get in the habitat while, on the other
hand, plants of Hffgla. cinnabarina tend to get
shorter as they don’t have to elongate
the pseudobulbs to get light as they won’t be growing
inside shrubs.
2-
Inflorescence hight:
This have to be used very carefully. Although it is quite obvious that there are species with very short or very long inflorescences, it is also true that some species tend to produce shorter or longer inflorescences in cultivation (e.g. Hoffmannseggella itambana). This is especially problematic as a common trait recently is to do taxonomic work with cultivated plants (for whatever reason). Considering that species keys on the group frequently use inflorescence hight, one can imagine how wrong they can get.
.
 |
 |
|
Hoffmannseggella
itambana,
when in cultivation (left), tends to produce
much taller inflorescencesa than in the habitat
(right).
|
|
3-
Number of flowers:
We
have to be careful with this one. When Pabst described Laelia
liliputiana, he wrote that the main feature
was that the species ALWAYS produce ONE flower
on the inflorescence. Today we know not to make statements
like these.
 |
Hoffmannseggella liliputiana, here with
three flowers on an inflorescence. This is quite
rare but, in the habitat, two flowers is quite common
on an inflorescence. If we take literally what the
protologue says, we would have another species here.
|
D- There are other misconceptions that, although not frequently or ever used in keys, give us an incorrect idea of the species of the group. Among these we have:
1-
Plants always come from high elevation mountains:
At
least two species can be found on the coast, and one at around
100 m. elevation (e.g. Hoffmannseggella
gloedeniana).
|
|
Hoffmannseggella gloedeniana, hardly a high elevation species.... |
2-
Plants are cool growers:
Well,
besides the ones on item 1, even the ones growing in the
mountains can be subject to
very high temperatures during the day, and not always
gets cold during the night (e.g. Hoffmannseggella
briegeri).
E- Now there are features that are quite stable,
besides the flowers of course, and these are very useful
for identification. Among these we have:
1- Plant habit:
This
is a very solid feature, which means that at least some
species can be separted just by that. This is actually
one of the most important features to separate Hoffmannsegella
crispata from Hffgla.
flavasulina, for example. That means that they can
be told apart just by
looking at the plants.
2- Inflorescence habit:
Another
quite stable feature, if one knows what to look for. One
of the most important things here is flower distribution.
Species that have the flowers bunched at the top will
always be that way.
A good example is in the Hoffmannseggella
crispata group.
Hoffmannseggella crispata can be easily separated
from Hffgla. endsfeldzii because it has the
flowers crowded at the top while Hffgla. endsfeldzii has
them spread. Even if everything
else was equal, that would be enough to separate them..
 |
 |
|
Here
we have Hoffmannseggella
endsfelzii on the left and Hfglla. crispata on
the right.
It is easy to see the differences in flower placement on the inflorescence. |
|
Natural Hybrids and speciation
This
is a group of plants in active speciation, and this
can be seen by the quantity of natural hybrids. Whenever
more than one species grow together, there is a good possibility
that the natural hybrid between them will be found.
If
the flowering season of the species involved is different,
which is the norm, few hybrid plants are usually found
and they usually end up being diluted back into one of
the populations (introgression). Good examples are Hoffmannseggella
xCristinae and Hffgla. xRaganii.
This
also happens if the microhabitats are different, even when flowering
season is the same (e.g. Hoffmannseggella
xHispidula). It is easy to understand if we remember
that seeds are very heavy and thus have a very localized
dispersion.
However,
when different species share the microhabitat and have
similar flowering seasons, full hybrid populations can
be found (e.g. Hoffmannseggella xBritoi).
 |
 |
 |
 |
|
Here
we have Hoffmannseggella briegeri (top left)
and Hfglla. rupestris (bottom left), that
when
hibridized produce Hfglla. xCristinae (top and bottom right, flowers from two different plants). |
|
Hoffmannseggella species affinity list
This is tentative grouping of species in alliances within the genus. It is based in plant and flower morphology similarities. Species followed by (*) are still doubtful regarding placement. Species with names between (“”)s need new species names and are referred by their commonly but incorrect known name.
1- Yellow flowers (mostly)
Hoffmannseggella blumenscheinii alliance:
blumenscheinii
gracilis
Hoffmannseggella bradei alliance:
bradei
briegeri
esalqueana
itambana
kleberi
verboonenii
Hoffmannseggella cardimii alliance:
cardimii
Hoffmannseggella crispata alliance:
crispata (flava)
endsfeldzii
flavasulina
milleri
sanguiloba *
Hoffmannseggella mixta alliance:
alvaroana
bahiensis *
mixta
Hoffmannseggella gloedeniana alliance:
gloedeniana
macrobulbosa
2- Orange to red flowers
Hoffmannseggella angereri alliance:
angereri
Hoffmannseggella cinnabarina alliance:
cinnabarina
“cowanii”
colnagoi
hegeriana
mirandae
Hoffmannseggella harpophylla alliance:
brevicaulis
harpophylla
kautskyi
3- Pink to purple flowers
Hoffmannseggella caulescens alliance:
caulescens (crispilabia)
pabstii (mantiqueirae)
Hoffmannseggella duveenii alliance:
duveenii
Hoffmannseggella longipes alliance:
fournieri
ghillanyi
kettieana
liliputiana
longipes (lucasiana)
reginae
Hoffmannseggella munchowiana alliance:
munchowiana
Hoffmannseggella pfisteri alliance:
pfisteri
Hoffmannseggella rupestris alliance:
rupestris
tereticaulis
Comments on DNA studies
This
is a section where I voice my personal opinion on the
very newest trend in orchid science. As with something
new, a lot of people jump in and get to the conclusion
that this is the solution for all our classification
problems. Some people go as far as to say that we should
throw all the previous knowledge away and start anew.
I lived enough to remember that exactly the same thing
happened when cromossome studies were new and also phytochemistry
was new. Eventually, all this information was added
to our common knowledge, together with morphology, anatomy,
phytogeography and several other study areas. The more
info we have, the more precise can our conclusions be.
DNA studies, being so new, of course will have changing
results as a consequence of evolving methodology. Following
are parts of Cassio van den Berg’s excellent essay “Considerações
sobre as ex-Laelias brasileiras, Sophronitis e outros
gêneros”, that was published online (van den
Berg, 2003). I choose a few paragraphs that I thought
were more relevant for the discussion and commented after
each of them. Of course these can be taken out of context
so I strongly recommend everyone to read the whole of
it, it is excellent reading. So here we go.
“The
first is that, although the ITS data show clearly that
the Brazilian species of Laelia had
to be segregated, the level of variation (the differences)
in the DNA within the trees was low, and consequently,
the subgroups formed within the tree had little statistic
confidence. This means that in a next DNA study (see below
for an example), the relationships among the different
groups of Laelia could change reasonably. Therefore
from a stability of names viewpoint (to avoid later changes)
making a single genus was a safer option;”
- Agreed, and this is reason enough to consider any
conclusions as premature although as more info is added
the better the system works.
“The
second important point, is that maintaining these species
in a single group we have more information in the system.
For example, in the classification system of orchids most
largely used today (Dressler, 1993), there are no categories
between subtribe and genus, and consequently, the genera
are listed in alphabetical order within subtribes. If we
had proposed several genera for these species, these genera
would be lost among other 50 other genera of Laeliinae,
however, the information that all these species are related
would have been lost. Our intention is later to propose
subgenera which show the different groups of species, which
is a formal way of showing the subgroup information. However,
because the studies are still in course and we could not
achieve conclusive delimitation of the subgroups, these
proposals were still not done, in agreement with the stability
factor;”
- Agreed with the first part, but this shows how initial
the conclusions were at this point.
“The
third factor is of historical nature: traditionally
it has been maintained in the literature that an average
size of a group (family, genus, etc.) is around 40 items,
and this was one of the criteria used in the flowering
plants classification of APG (1998). The Brazilian species
of Laelia were a little above this number, but
on the other hand the division into smaller genera would
produce many genera with much less species (around 10),
and some monotypic genera. Furthermore, the genus Laelia was
well accepted in the size it was, only with the questions
regarding the two groups (the Brazilian and Mexican).
The subgenera of Laelia were always a good solution
to show the subgroups, but without losing the coherence
of the larger group;”
-
Agreed in essence, but keeping polyphylletic groups
together doesn’t make much sense. And Hoffmannseggella for
example has the perfect 40 species number, give or take a
couple.
“And
finally, attempts of splitting the subgroups into distinct
genera had already been done (ex. Hoffmannseggella,
Jones, 1968) and were not well accepted by both botanists
(Garay, 1973) or orchid growers. In this way, when we
chose to transfer all species of this group to Sophronitis,
we chose the simpler, stabler system with the best information
content.”
-
Yes, Jones didn’t do a good job, but he didn’t
know the group and its species very well (or at all).
So considering everything, his work would face an uphill
battle on taxonomic circles no matter what. As for the
amateurs, taxonomists can do whatever they want, Laelia
purpurata will always be Laelia purpurata.
Until, of course, all the old school growers die and
new ones never know about the old names.
“In
the work of Chiron and Castro (2002), although a morphological
justification is given, a careful reading makes it clear
that all groups were delimited exclusively based on
the trees of van den Berg et al., (2000), which were
reproduced therein, and also the list of species does
not differ from the list published in Sophronitis in
our adjacent paper. No additional data was presented to
justify the decisions. This somehow made us to worry,
because we knew the fragility of ITS data to establish
groups within Sophronitis (this fragility was stressed
in the original study of DNA). This is even more surprising
because in the beginning of their article, Chiron and
Castro (2002) cited several preliminary cautions about
the use of phylogenetic trees for establishing classifications
(items A-E), and immediately ignored these criteria (especially
item E about robustness of branches) in their proposal.”
-
See, Cassio nailed the problem here. For
his conclusions to be contested, the work has
to be done again, that’s
why we have “Materials and Methods” sections
on scientific works – to allow repeatibility.
If you don’t redo the
work to get to your own conclusions, you risk basing
your arguments in data YOU DON’T HAVE, and in
the end
we have an esoteric discussion of sorts. What I tried
to do here is circunsbcribe Hoffmannseggella purely
on
morphological and ecological grounds, and I think
such an uniform group is very easy to deal this way.
“Although
studies with several other DNA regions for assessing
relationships within Sophronitis are
not finished, preliminary data with two plastid regions
(Fig. 1) already show that in order to use the system
of Chiron and Castro (2002), which was just proposed,
several substantial modifications would be necessary”.
“Based
on data from the regions trnL-F and matK, the species
of Sophronitis in the old sense (before
receiving the species of Laelia), constitute a
good group (differently from the separation of S. cernua which
appeared in the ITS trees), and therefore the placement
of the group of S. coccinea in Hadrolaelia is inadequate.
Similarly, S. harpophylla goes as sister to the
rupicolous species, what makes a dubious situation. At
the same time that these species placed in Dungsia have
some differences in relation to the rupicolous species
(especially size), it is obvious that they share many
other morphological characters with this group, and in
this sense it appears better than Dungsia would
be lumped to Hoffmannseggella in their system.
In this case the only real justification that could be
made to keep Dungsia separated from Hoffmannseggella would
be the will of the authors.”
- Well, that sums it up.
Conclusion
With the above, I have to conclude that:
1-
I cannot accept Dungsia, first because
it was described based on the first ITS sequence work
that is being contradicted by newer studies, but more
importantly because a key morphology feature used to
justify it is plain wrong. All the species of the group
DO have floral spathe. And based on morphology, just
look at the flowers and you know where those species
belong.
2-
I am glad this is such an easy group to delimit morphologically.
Taxonomists have been studying morphology for more
than 200 years for the simple reason that it is so
easy to study what you can see. This was the only
option in the beginning and now there is so much information
that it just can’t be dismissed
by the shake of the hand to start from square 1. We
also have to remember that:
Phenotype
= Genotype + Environment, which
can be translated as “morphology is a
consequence of the DNA plus environmental factors”.
3-
DNA studies, at this stage, are very preliminary,
and I can’t rely on them to get to conclusions
on group separations. I won’t get into detailed
discussions for several reasons, including the fact
that I didn’t do the work and so have no info
to bring to the table and especially because it doesn’t
matter as I already made up my mind that they are still
preliminary. I took a hard look at all of the DNA work
being done and find it extremely interesting, so will
get back to it in the future to see how things are going.
You see, the more I think of it I imagine that, even
a long time from now, the molecular and morphological
info will converge to a point that the results will
be essentially the same. This is because both are just
two different ways to look at the same thing.
4- Talking with Cassio during the timeframe of the
WOC, we discussed his opinion that it will be better
to consider the Brazilian Laeliinae as part of Cattleya and
the different genera as subgenera of it. I think this
is a sensible approach and addresses the main problems
created with lumping them with Sophronitis and
makes it basically a matter of splitting or lumping
genera. In this particular case, lumping is an option
but it get at odds with what is happening with other
groups as for example the Oncidiinae and Pleurothallidinae where
heavy splitting is happening. The rupicolous laelias
are a very uniform group that will stay equally well
as a separate genus or a subgenus of Cattleya so
I will be watching what happens.
5-
And finally, at the end of the day, we all have our
personal opinions and these reflect on every work
we do. Everything I am saying here is just that, my
PERSONAL OPINION. The same way I don’t agree
with something, you are of course free to disagree with
me. Regardless of how much information I tried to put
together, it doesn’t change that fact.
 |
Hoffmannseggella harpophylla in
the natural habitat, prope Venda Nova do Imigrante,
ES. It is easy to see the well-developed
inflorescence sheaths, typical of all species in
the genus. This by itself renders invalid the morphological
justification for Dungsia.
|
|
|
|
Bibliography:
Jones, H.G. 1968. Acta Botanica Academiae Scientiarum
Hungaricae 14: 69.
Lindley, J. 1842. SECT. Parviflorae in Bot.
Reg., XXVIII, sub. t. 62.
Pabst,
G.F.J. & Dungs,
F. 1975. Orchidaceae Brasiliensis, Band I, p. 146. Brucke-Verlag,
DE.
Schlechter, R. 1917. Die Einteilung der Gattung Laelia und
die Geographische Verbreitung ihrer Gruppen. Orchis 11:87-96.
van den Berg, C. and M.W. Chase. 2000. Nomenclatural
notes on Laeliinae 1. Lindleyana 15(2):
115–119.
van
den Berg, C. 2003. Considerações sobre
as ex-Laelias brasileiras, Sophronitis e outros
gêneros. Orchid News 20 in Brazilian Orchids online
(http://www.delfinadearaujo.com)
van den Berg, C. and M.W. Chase. 2004. A chronological
view of Laeliinae taxonomical history. Orchid
Digest 68(4): 226-254.
An exercise in personal opinion
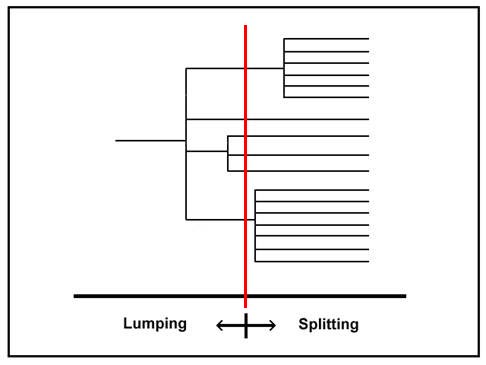
This is a simple random diagram to show how these things go. Depending on the person looking at it, the red line can slide left or right. Depending on where it is positioned, we go from one group to four, six, twelve or seventeen. All this without changing the data, just the person looking at it… And this is valid for morphological data, numeric taxonomy data, and even… DNA data. Just put a real key instead of this one and see people fighting over where to put the line. Human nature, but I digress.
Photos: Francisco Miranda
Francisco
E. Miranda
4763 Polk City Road,
Haines City, FL 33844, USA
fmiranda@att.net
|
Any
kind of reproduction (print, digital or anyone)
of any type of material of this site: texts,
layout, photos, images and others - is
strictly forbidden without previous written permission of the authors. Any solicitation or information by the e-mail:bo@sergioaraujo.com |
